Our history
For more than 20 years, Biogaran has been innovating and developing itself to provide you with services and therapeutic solutions that answer your needs.
Discover our history.

Biogaran, reinventing itself since 1996
-

-

-

-

-

-

-

-

-

Our international development
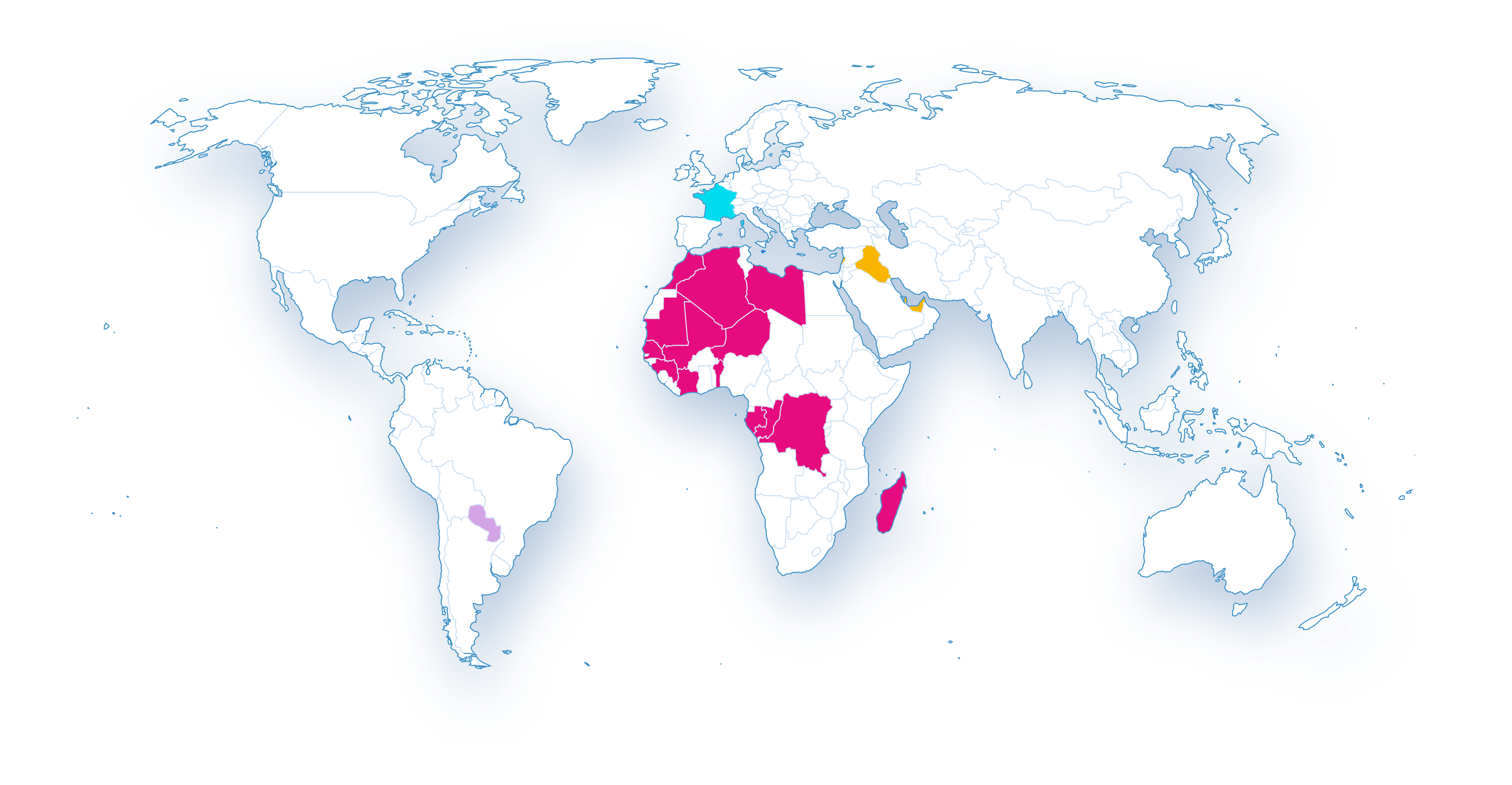
- Africa
- Near and Middle East
- Latin America
- Europe
-

-

-

-

-

-

- 2023 Beginning of our operations in Georgia
- 2024 Beginning of our operations in Kuwait

The development of our activities in the Near and Middle East
It is with the goal of offering quality medicines that meet the strictest European standards that Biogaran began the deployment of its activities in the Near and Middle East in 2017 with the launch of its commercial activities in Lebanon. In order to meet the challenges of public health and to provide quality medicines adapted to the needs of patients, Biogaran wishes to continue to develop its activities in the region in the coming years, particularly in Iraq, where Biogaran will launch its activities in 2022.
Thanks to bioequivalent quality medicines complying with the most stringent European standards, Biogaran's ambition is to become the first choice for patients who wish to have access to high-quality generic drugs and that they will be able to use safely.
